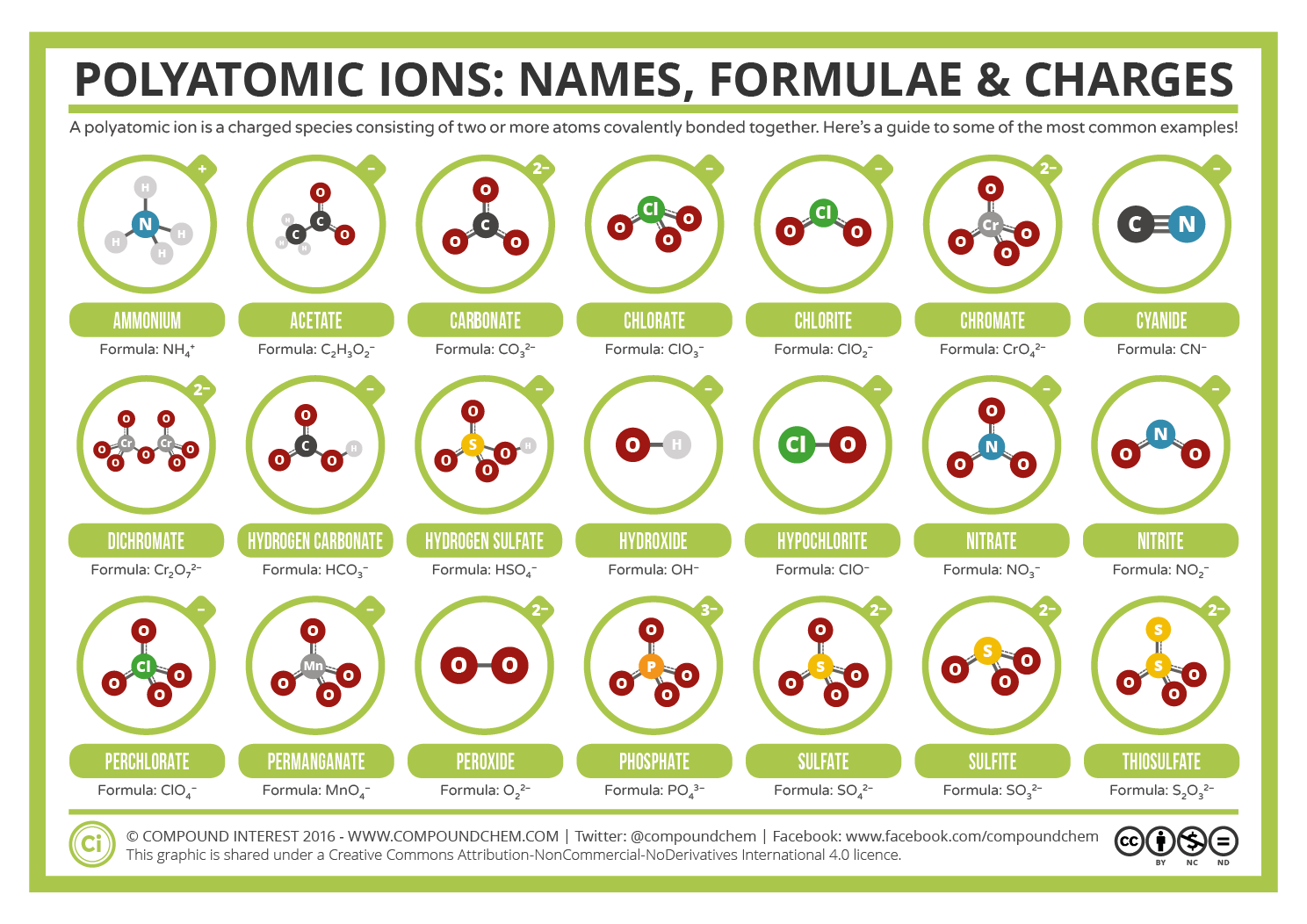
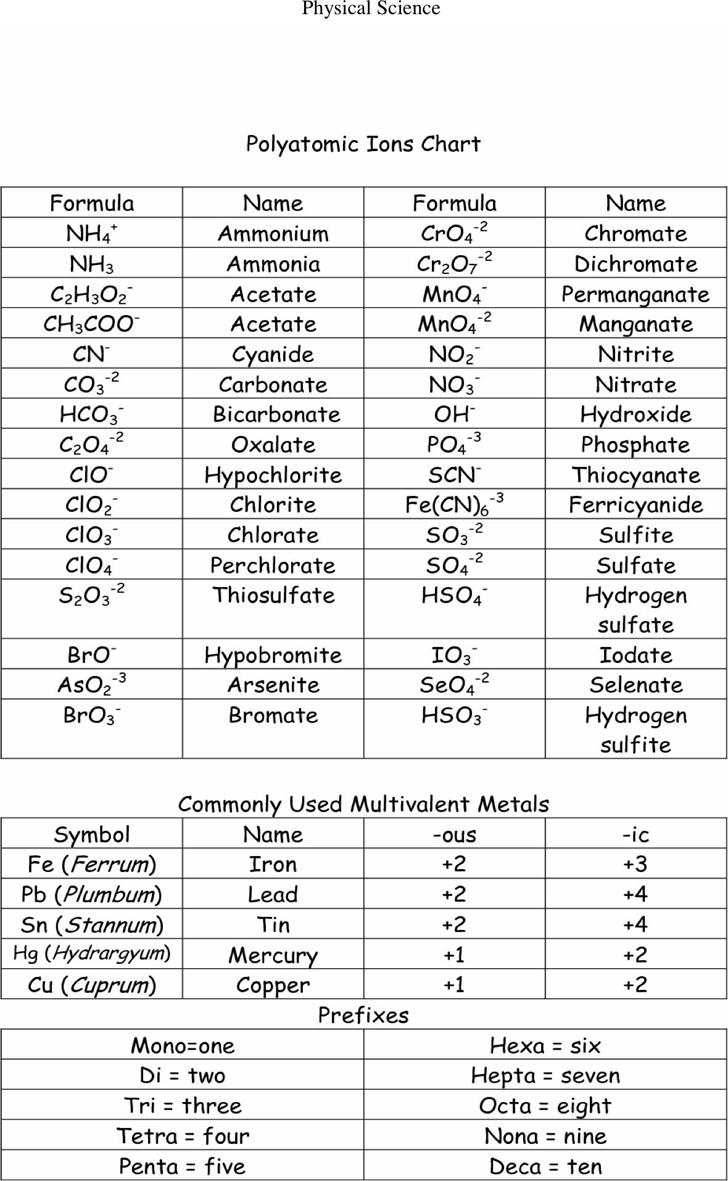
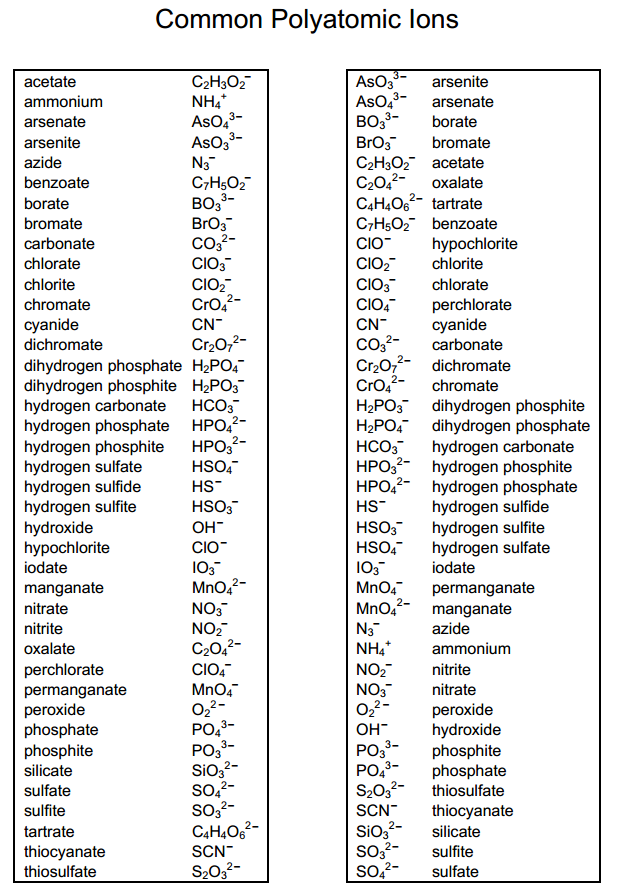
A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web table \(\pageindex{1}\) lists the ion names and ion formulas of the most common polyatomic ions. Acetate bromate chlorate chlorite cyanide hydride hydrogen carbonate (bicarbonate) hydrogen sulfate (bisulfate) hydroxide hypochlorite iodate nitrate nitrite perchlorate permanganate thiocyanate. Web this is a list of some of the most common polyatomic ions.
A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. Here's a guide to some of the most common examples! Web this polyatomic ions list contains many common polyatomic ions grouped by charge.
Web this is a list of some of the most common polyatomic ions. Acetate bromate chlorate chlorite cyanide hydride hydrogen carbonate (bicarbonate) hydrogen sulfate (bisulfate) hydroxide hypochlorite iodate nitrate nitrite perchlorate permanganate thiocyanate. A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Each entry contains the ion's name, molecular formula and chemical structure. The following table lists some of the common polyatomic ions.
Web table \(\pageindex{1}\) lists the ion names and ion formulas of the most common polyatomic ions. Each entry contains the ion's name, molecular formula and chemical structure. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. It has one nitrogen atom and three oxygen atoms and an overall −1 charge. The following table lists some of the common polyatomic ions. For example, \(\ce{no_3^{−}}\) is the nitrate ion; Web this is a list of some of the most common polyatomic ions. Here's a guide to some of the most common examples! Web being familiar with the names, charges, and formulas of the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity. Acetate bromate chlorate chlorite cyanide hydride hydrogen carbonate (bicarbonate) hydrogen sulfate (bisulfate) hydroxide hypochlorite iodate nitrate nitrite perchlorate permanganate thiocyanate. Web a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web this polyatomic ions list contains many common polyatomic ions grouped by charge. Here's a guide to some of the most common examples! A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web common polyatomic ions ;
Web A Polyatomic Ion Is A Charged Species Consisting Of Two Or More Atoms Covalently Bonded Together.
Web being familiar with the names, charges, and formulas of the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity. A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. The following table lists some of the common polyatomic ions. Web table \(\pageindex{1}\) lists the ion names and ion formulas of the most common polyatomic ions.
Acetate Bromate Chlorate Chlorite Cyanide Hydride Hydrogen Carbonate (Bicarbonate) Hydrogen Sulfate (Bisulfate) Hydroxide Hypochlorite Iodate Nitrate Nitrite Perchlorate Permanganate Thiocyanate.
Web this is a list of some of the most common polyatomic ions. Each entry contains the ion's name, molecular formula and chemical structure. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. It has one nitrogen atom and three oxygen atoms and an overall −1 charge.
Here's A Guide To Some Of The Most Common Examples!
Web common polyatomic ions ; Here's a guide to some of the most common examples! Web this polyatomic ions list contains many common polyatomic ions grouped by charge. For example, \(\ce{no_3^{−}}\) is the nitrate ion;