Values for p c and t c for various substances can be found in table c.12. Web properties of common gases. Web the compressibility factor equation can be written as: Is the same for all gases. The ideal gas equation (eq.
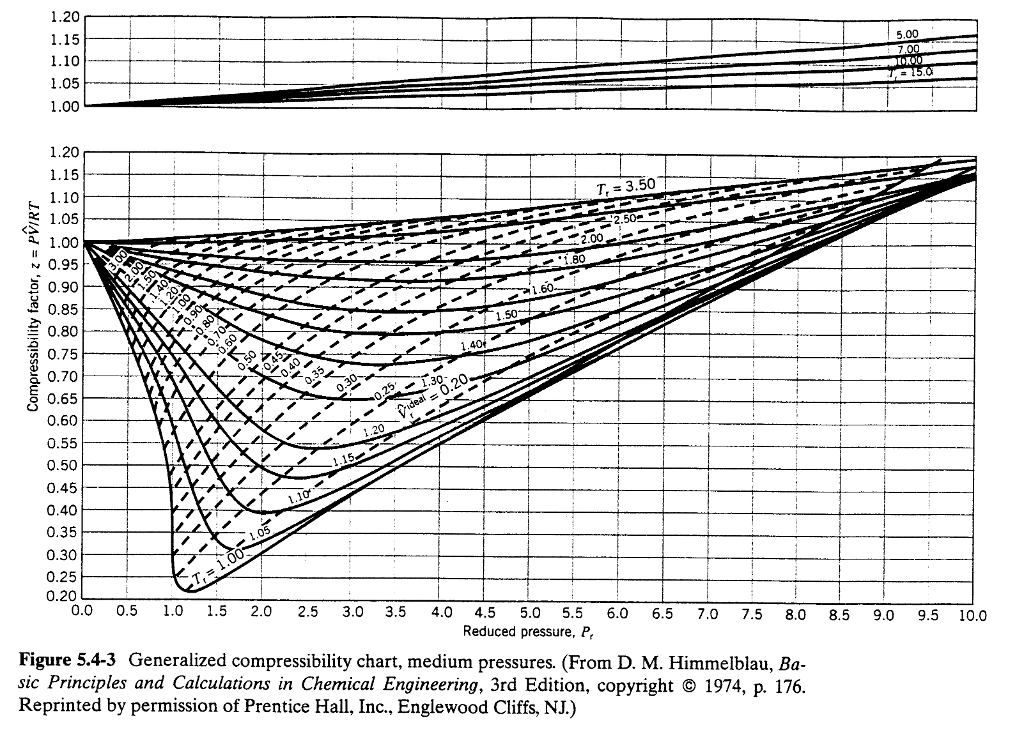
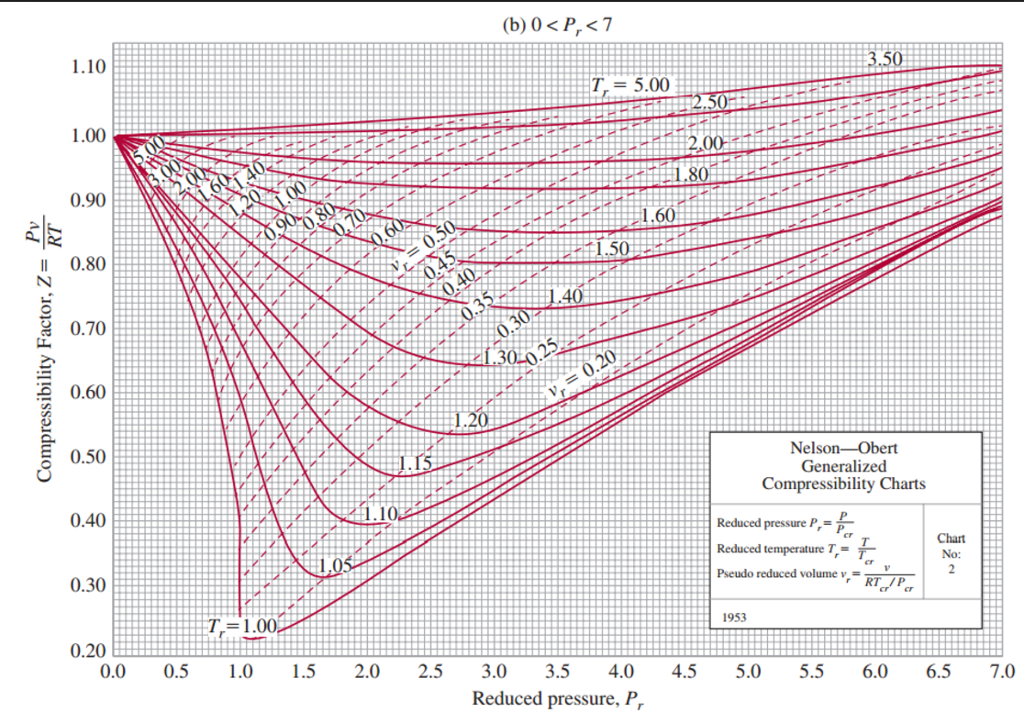
If we only know the temperature and pressure, we can still calculate it using a compressibility chart. Web figure 1 shows the essential features of a generalized compressibility factor chart. It is valid for many substances, especially those that have simple molecular structures. Web properties of common gases. Web the generalized compressibility chart can be viewed as a graphical representation of the gas behaviour over a wide range of pressures and temperatures.
Example of a generalized compressibility factor graph (public domain; Web the generalized compressibility chart can be viewed as a graphical representation of the gas behaviour over a wide range of pressures and temperatures. Web the compressibility factor chart plots the compressibility factor , equal to , where is the volume per mole, versus the reduced pressure for several values of the reduced temperature. Is the same for all gases. Web the resulting z = z (p r, t r, v′ r) plot is now called the generalized compressibility chart and is shown in figures 11.5, 11.6, and 11.7.
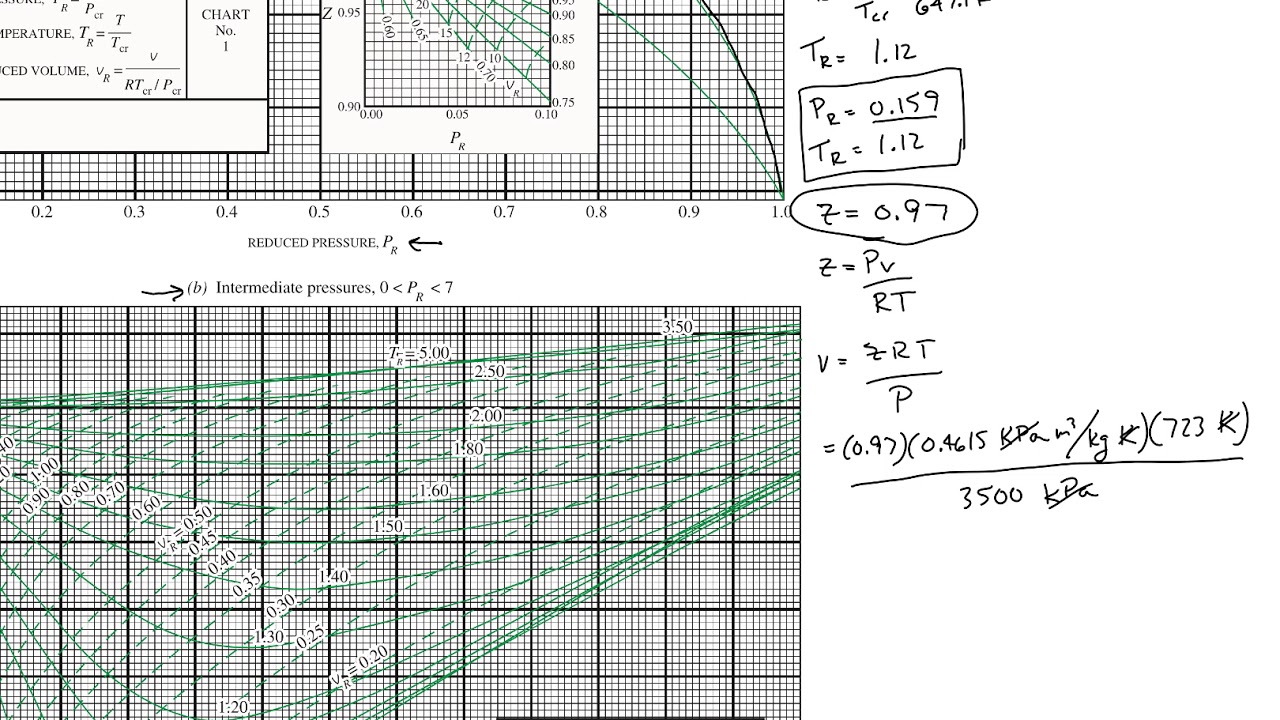
At high temperatures (tr > 2), ideal gas behavior can be assumed with good accuracy. The ideal gas equation (eq. Reduced pressure is the ratio of the actual pressure. Web 13.5.1 generalized compressibility chart. Web the compressibility factor equation can be written as: Then, a compressibility factor (z) can be used to quantify If we only know the temperature and pressure, we can still calculate it using a compressibility chart. For air at 200 k, 132 bar, tr = 200 k/133 k = 1.5, pr = 132 bar/37.7 bar =. The ideal gas equation (eqs. Web properties of common gases. Vapor pressure curves for common pure gases. Compare the results of parts (a) and (b) with values obtained from the thermodynamic table or software11. Values for p c and t c for various substances can be found in table c.12. A practical guide to compressor technology, second edition, by heinz p. On a generalized compressibility chart, the compressibility z z is plotted as a function f = f(pr,tr) f = f ( p r, t r) of the reduced pressure and temperature.
At Very Low Pressure (Pr << 1), Gases Behave As An Ideal Gas Regardless Of Temperature.
The ideal gas equation (eq. A practical guide to compressor technology, second edition, by heinz p. Web the compressibility factor chart plots the compressibility factor , equal to , where is the volume per mole, versus the reduced pressure for several values of the reduced temperature. Web the generalized compressibility factor chart shows how the value of {eq}z {/eq} fluctuates in regard to reduced pressure and temperature.
A Test For Whether A Gas Behaves Ideally Can Be Obtained By Comparing The Actual Pressure And Temperature To The Critical Pressure And Temperature.
Web generalized compressibility chart and the compressibility factor, z. The reduced pressure and temperature are defined by and , respectively, where is the critical pressure and is the critical temperature. Then, a compressibility factor (z) can be used to quantify Bloch copyright © 2006 john wiley & sons, inc.
Compare The Results Of Parts (A) And (B) With Values Obtained From The Thermodynamic Table Or Software11.
Example of a generalized compressibility factor graph (public domain; The ideal gas equation (eqs. Web essentially it corrects for the deviation of a real gas from an ideal gas. If we only know the temperature and pressure, we can still calculate it using a compressibility chart.
Z = Pv¯¯¯¯ Rt Z = P V ¯ R T.
Values for p c and t c for various substances can be found in table c.12. Web properties of common gases. Z = p × v / n × r × t, where z is the compressibility factor, for pressure p, volume v, gas constant r, number of moles n, and temperature t. Web the resulting z = z (p r, t r, v′ r) plot is now called the generalized compressibility chart and is shown in figures 11.5, 11.6, and 11.7.