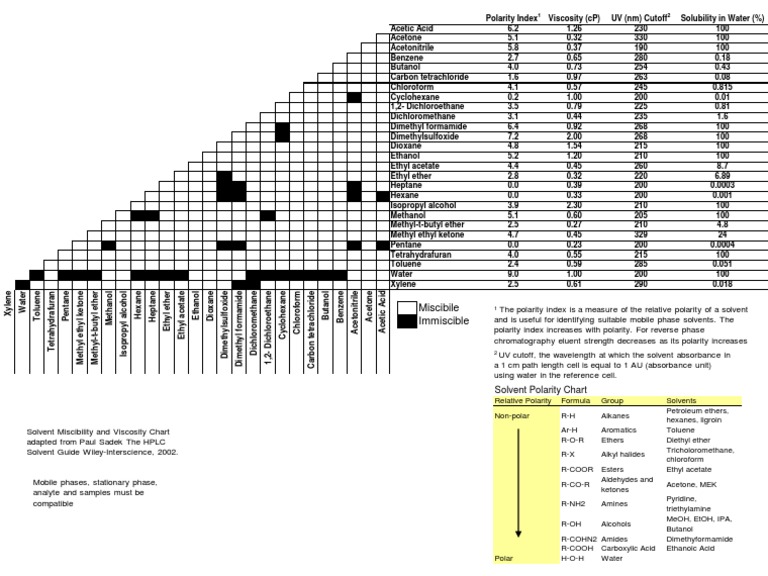
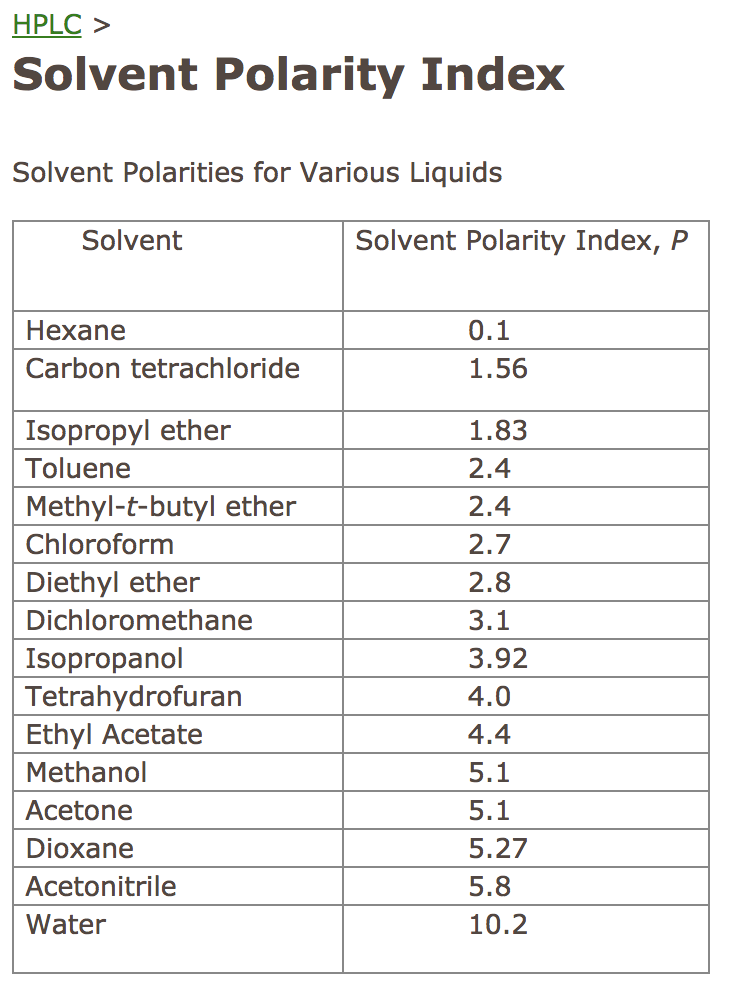
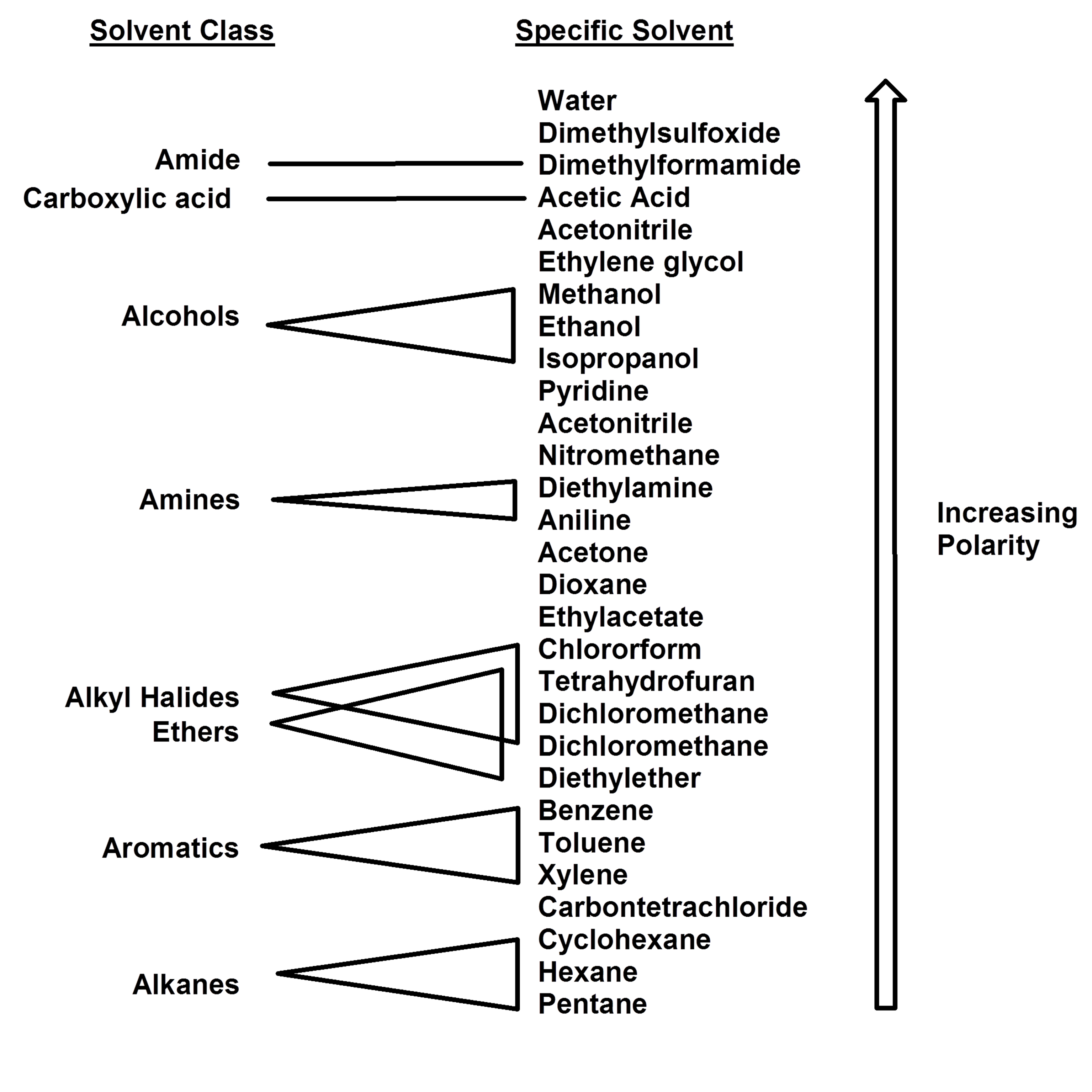
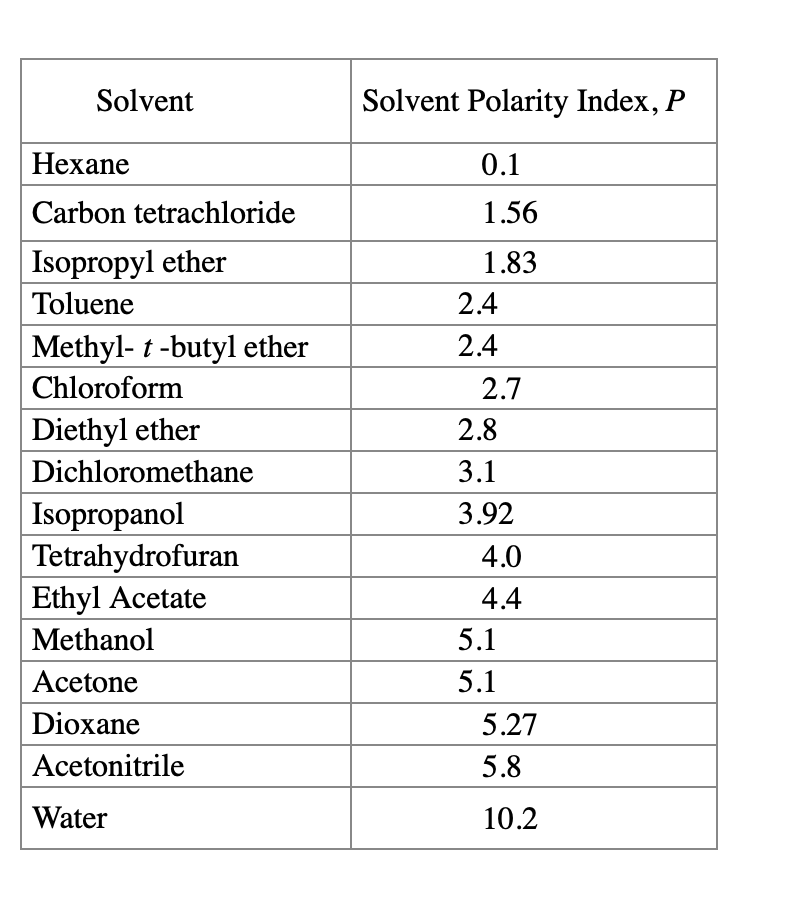
Common solvents arranged from the least polar to the most polar This page uses frames, but your browser doesn't support them. Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. Bond dipole moment measurements can be found on individual solvent pages in our solvent center.
Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Demystifying synthetic organic chemistry since 2004. Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. Bond dipole moment measurements can be found on individual solvent pages in our solvent center.
Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Demystifying synthetic organic chemistry since 2004. Laboratory techniques and methods to improve your experimental skills. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. Web chemists approximate the vector sum of a molecule's bond dipole moments to be an estimate of the molecule's polarity.
Common solvents arranged from the least polar to the most polar Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. This page uses frames, but your browser doesn't support them. Demystifying synthetic organic chemistry since 2004. Web chemists approximate the vector sum of a molecule's bond dipole moments to be an estimate of the molecule's polarity. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethyl ether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. Bond dipole moment measurements can be found on individual solvent pages in our solvent center. Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Laboratory techniques and methods to improve your experimental skills.
Web Chemists Approximate The Vector Sum Of A Molecule's Bond Dipole Moments To Be An Estimate Of The Molecule's Polarity.
Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethylether benzene toluene xylene carbontetrachloride cyclohexane petroleum. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane tetrahydrofuran dicholoromethane chloroform diethyl ether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether. Web information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Laboratory techniques and methods to improve your experimental skills.
Demystifying Synthetic Organic Chemistry Since 2004.
Bond dipole moment measurements can be found on individual solvent pages in our solvent center. Common solvents arranged from the least polar to the most polar This page uses frames, but your browser doesn't support them.