Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of water in terms of kpa, psi, mmhg, bar, atm, torr. Vapor pressure is measured in the standard units of pressure. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. At its boiling point (100 ° c), the vapor pressure of water is 658.0 torr (atmospheric pressure). The pressure up cancels the pressure down and boiling begins.
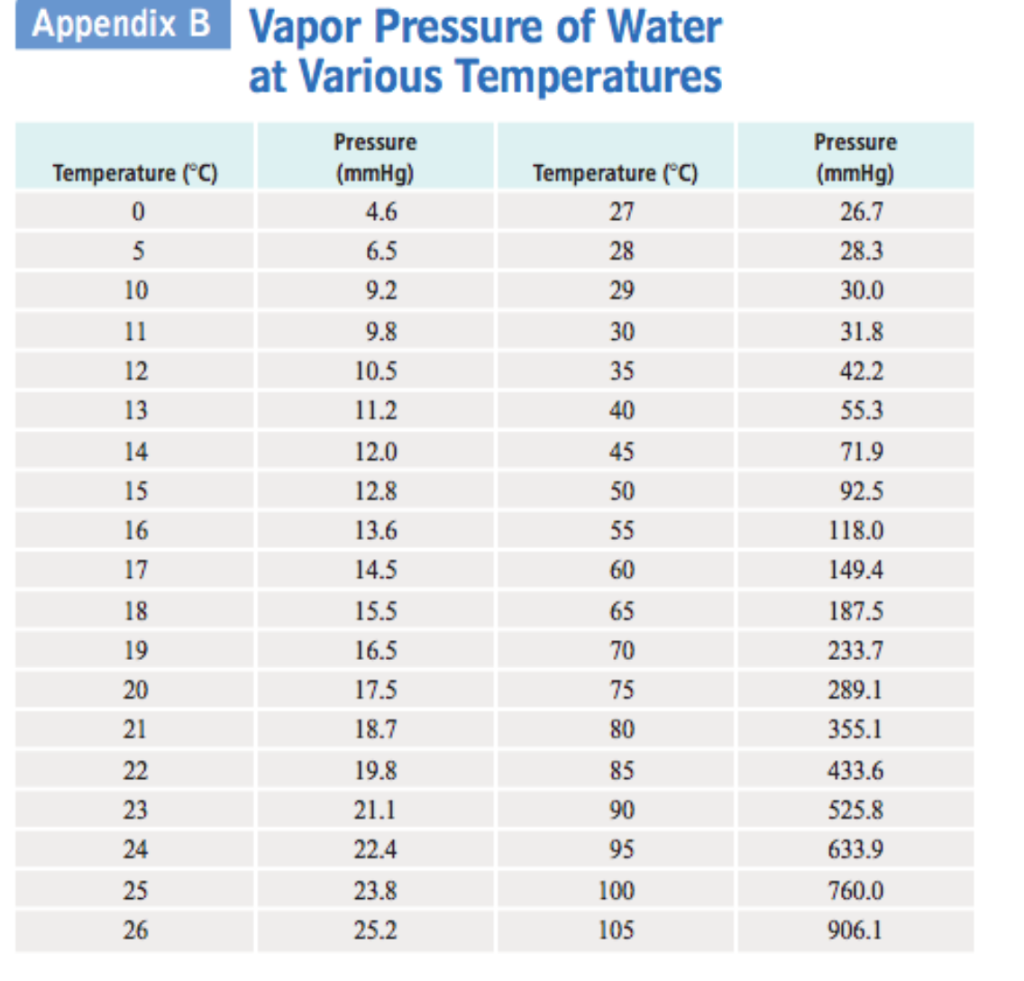
If you want the saturated vapor pressure enter the air temperature: Vapor pressure is measured in the standard units of pressure. At its freezing point (0 ° c), the vapor pressure of water is 4.6 torr. Atomic parameters (ie, ea, d,.) thermodynamic data. Enter a temperature or a dewpoint or both:
Web water vapour pressure table at different temperatures. Search search is the most efficient way to navigate the engineering toolbox. Web the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); At its freezing point (0 ° c), the vapor pressure of water is 4.6 torr. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air).
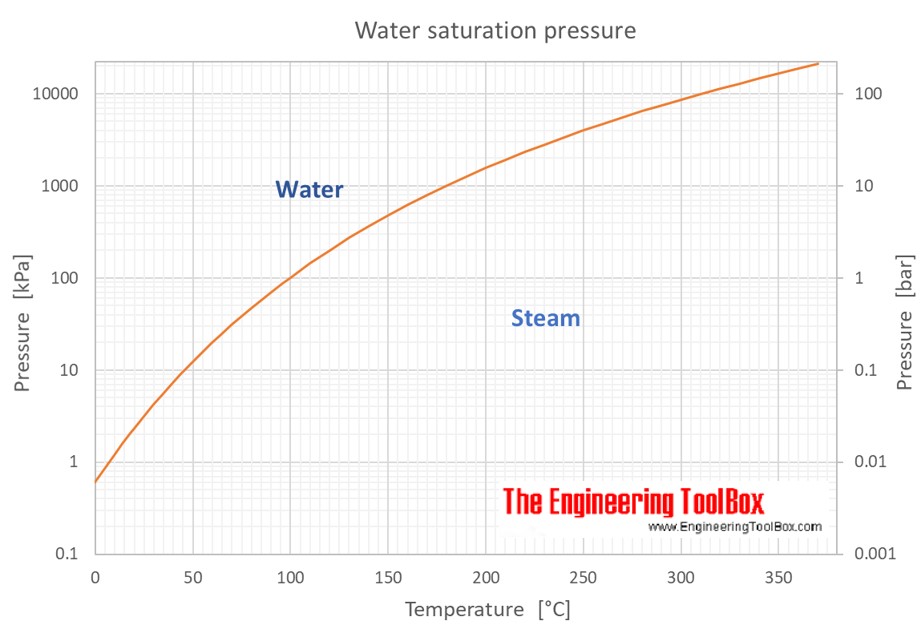
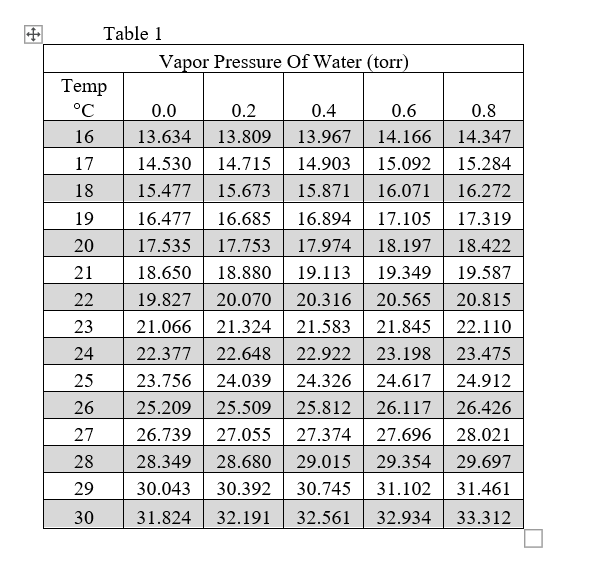
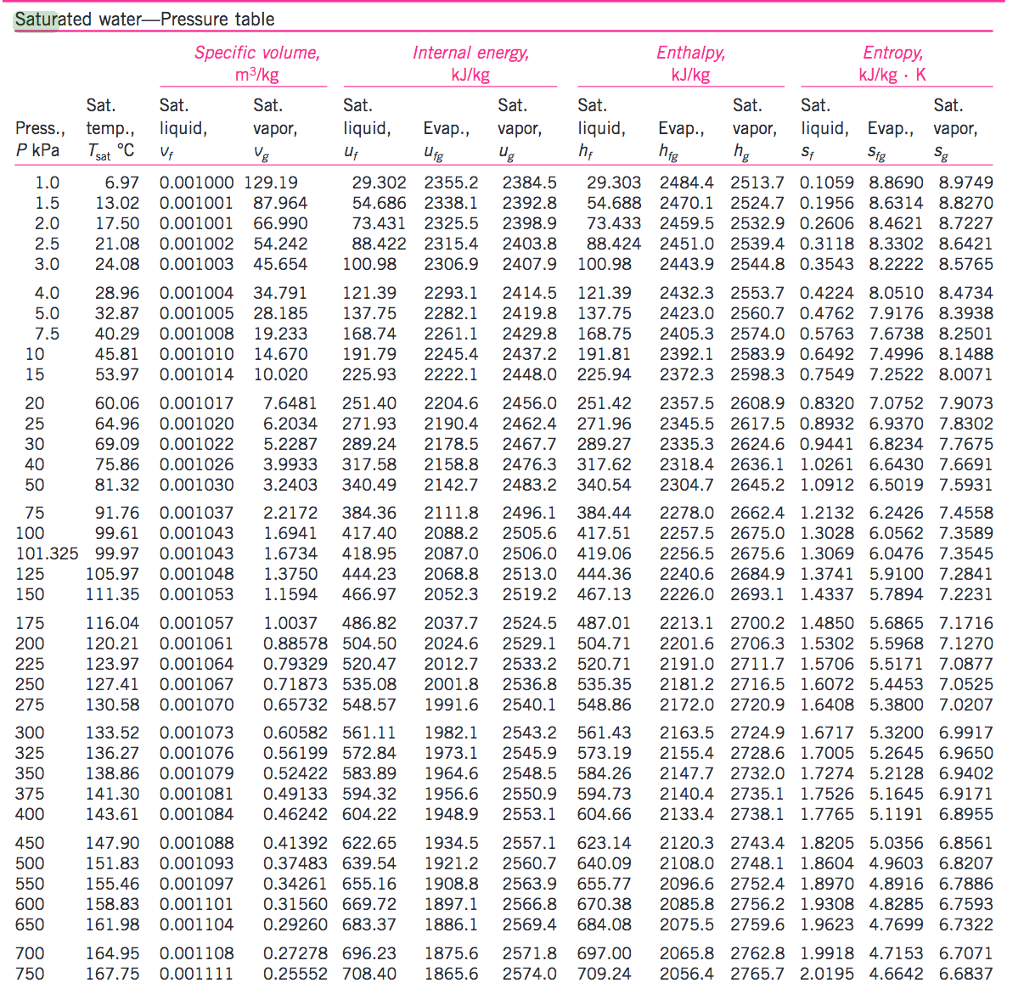
Web vapor pressure of h 2 o at various temperatures (celsius) note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Web what is the vapor pressure of a solution made by dissolving 100 grams of glucose (c 6 h 12 o 6) in 500 grams of water? The vapor pressure of pure water is 47.1 torr at 37 °c. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. Web vapor pressure of water from 0 °c to 100 °c. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. Atomic parameters (ie, ea, d,.) thermodynamic data. Water at high pressure has a higher boiling point than when that water is at atmospheric pressure. Vapor pressure of water at various temperatures. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Web water vapour pressure table at different temperatures. Generally a substance's vapor pressure increases as temperature. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. Search search is the most efficient way to navigate the engineering toolbox.
That Is, The Pressure Of The Vapor Resulting From Evaporation Of A Liquid (Or Solid) Above A Sample Of The Liquid (Or Solid) In A Closed Container.
The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and imperial units. The vapor pressure of pure water is 47.1 torr at 37 °c. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c.
Water At High Pressure Has A Higher Boiling Point Than When That Water Is At Atmospheric Pressure.
We look at the 68°f example specifically. Enter a temperature or a dewpoint or both: Crc handbook of chemistry and physics, 84th edition (2004). Atomic parameters (ie, ea, d,.) thermodynamic data.
Web The Vapor Pressure Of A Liquid Is The Equilibrium Pressure Of A Vapor Above Its Liquid (Or Solid);
By tim brice and todd hall. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa of argon, totaling 102.2 kpa, making the basis for standard atmospheric pressure. It is the pressure exerted by the saturated vapour in contact with the surface of the liquid at that temperature. The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and the water changes into a vapor.
Web For Example, As Water Boils At Sea Level, Its Vapor Pressure Is 1 Atmosphere Because The External Pressure Is Also 1 Atmosphere.
If you want the saturated vapor pressure enter the air temperature: Vapor pressure of water is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). Web water boiling temperature vs pressure in vacuum table chart.